What is the problem?
Stryker is conducting a Critical Product Correction of Pad-Paks used with the HeartSine samaritan Public Access Defibrillator (PAD), with an expiry date range between 17 April 2025 to 01 August 2029.
The affected Pad-Paks may not work due to bent locator pins and potential user error from improper insertion of Pad-Paks.
|
|
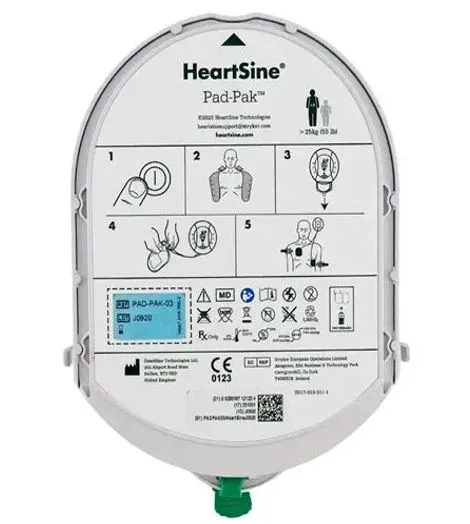
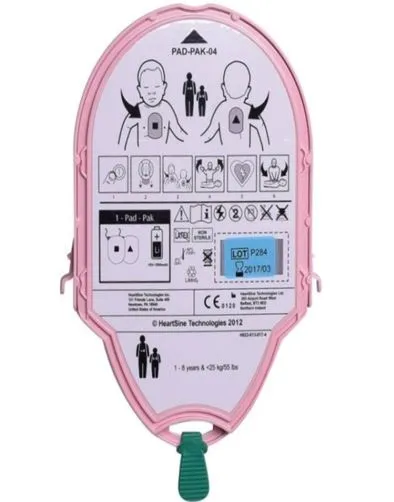
ARTG | Pad-Pak Catalogue Number | Product Description | Pad-Pak Expiry Dates | HeartSine Catalogue Number | HeartSine Product Model |
|---|---|---|---|---|---|
(Your Pad-Pak may be installed in this device) | |||||
338960, 312730 | PADPAK03 PADPAK04 PADPAK07 | Battery and electrode cartridge for HeartSine samaritan PAD | 17-April-2025 to 01-August-2029 | 350BASUK10 | HeartSine SAM 350P |
360BASSJ10 360BASUK10 | HeartSine SAM 360P | ||||
500BASUK10 500BASUKGW | HeartSine SAM 500P | ||||
What are the risks?
If the Pad-Pak locator pins are bent or the Pad-Pak is not properly inserted into the HeartSine samaritan PAD the device may fail to deliver therapy correctly.
This could lead to a delay in treatment or no treatment being delivered during use.
What should I do?
- Inspect any Pad-Paks to determine if the expiry date is between 17 April 2025 to 1 August 2029.
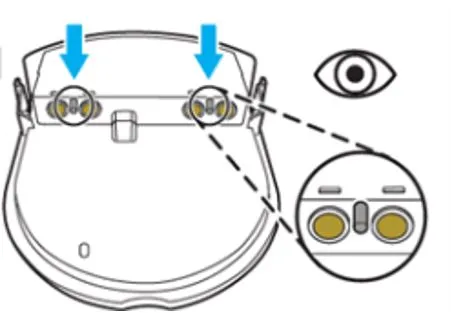
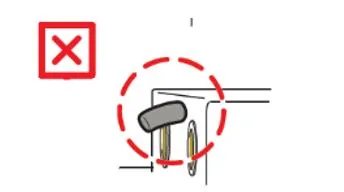
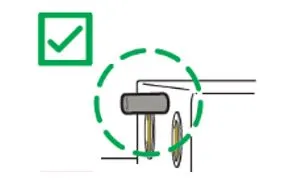
- If your Pad-Pak is within the expiry date range remove the Pad-Pak from the HeartSine samaritan PAD and check for bent locator pins (see images below).
- If locator pins are straight, re-insert the Pad-Pak following the user manual and you can continue to use the HeartSine samaritan PAD.
- If the locater pins are bent, remove that Pad-Pak from the HeartSine samaritan PAD, set it aside and contact Stryker on 02 8413 1250 or email padpak.ssp@stryker.com to organise replacement Pad-Paks.
- Once you receive the replacement Pad-Pak/s, please dispose of the affected Pad-Pak/s.
Further information
Please contact Stryker on 02 8413 1250 or email padpak.ssp@stryker.com for further information.
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices . Your report will contribute to our monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS) .
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.

