We will have limited operations from 15:00 Wednesday 24 December 2025 (AEDT) until Friday 2 January 2026. Find out how to contact us during the holiday period.
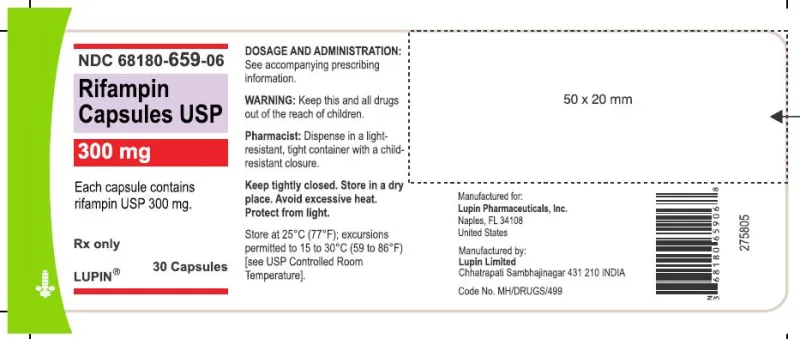
Rifampin (rifampicin) USP 300mg, 30 & 60 capsules (Lupin, USA)
Section 19A approved medicine
Rifampin (rifampicin) USP 300mg, 30 & 60 capsules (Lupin, USA)
Section 19A approval holder
Pro Pharmaceuticals Group Pty Ltd ABN 20 605 457 430
Phone
1300 077 674
Approved until
Status
Current
Medicines in short supply/unavailable
RIFADIN rifampicin 300mg capsules blister pack - ARTG 233443
RIMYCIN 300 rifampicin 300 mg capsule bottle - ARTG 48231
RIMYCIN 300 rifampicin 300 mg capsule bottle - ARTG 48231
Indication(s)
Tuberculosis: In the initial treatment and in re-treatment of patients with tuberculosis, it must be used in conjunction with at least one other antituberculosis drug.
Meningococcal Disease: Prophylaxis of meningococcal disease in close contacts of known cases and in carriers (not indicated for the treatment of meningococcal infections).
Images