What is the problem?
Following a serious adverse event, Invacare Australia is updating the Instructions for Use (IFU) of the Birdie Lifter and Compact Lifter. Users need to be aware of the correct position for the carabiner and the correct attachment of the hanger bar.
This action commenced in July 2025. Invacare is now attempting to reach a small number of customers who cannot be identified.

| ARTG number | Product name | Serial numbers |
|---|---|---|
| 166395 | Invacare Birdie Lifter and Invacare Birdie Compact Lifter | All |
What are the risks?
If the updated IFU and Maintenance checklist are not followed, or the carabiner is incorrectly attached to the lifter arm, the hanger bar may detach leading to a patient falling.
What should I do?
- Verify that the carabiner always remains in the correct assembly position.
- Images have been included in the updated IFU to show the correct and incorrect positions.
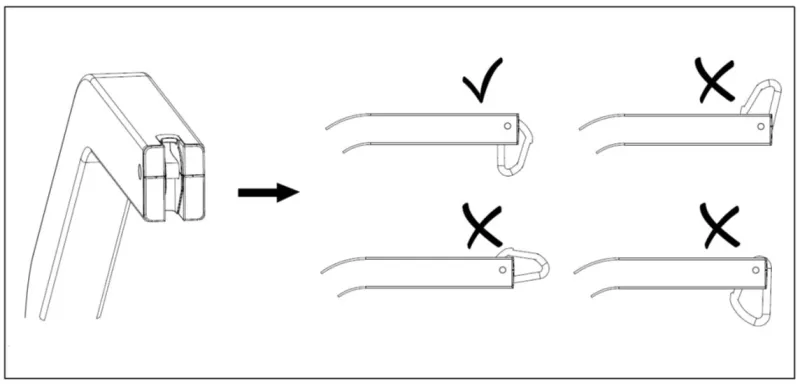
- The carabiner should not be able to rotate within the plastic parts where it is assembled without resistance. If unimpeded rotation occurs, this indicates the plastic parts are worn and should be replaced.
- An additional point to check the hanger bar and carabiner during regular maintenance has been added to the Maintenance Checklist.
- Contact Invacare Australia if you have any questions about these updates.
Further information
Please contact Invacare Australia on 1800 460 460 or email Compliance_ANZ@invacare.global for further information.
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to our monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS).
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.

