We will have limited operations from 15:00 Wednesday 24 December 2025 (AEDT) until Friday 2 January 2026. Find out how to contact us during the holiday period.
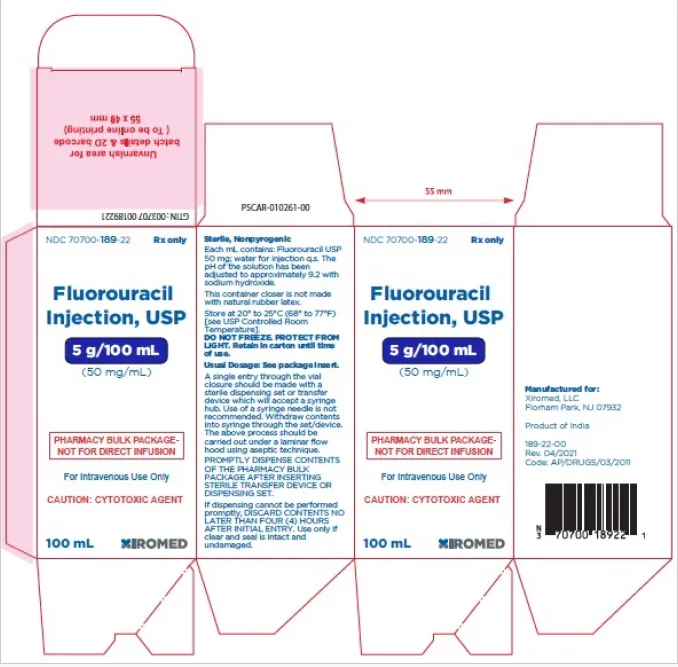
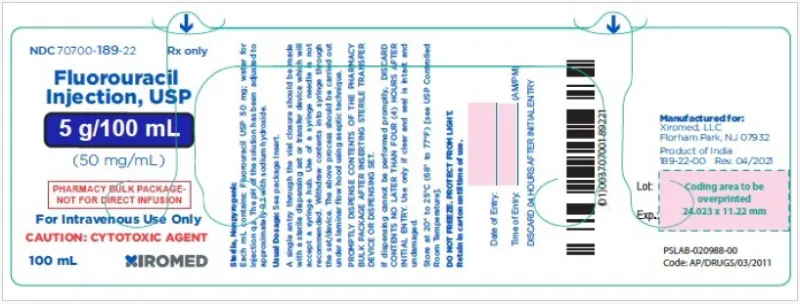
Fluorouracil Injection, USP 5g/100mL (50mg/mL) vial (Xiromed, USA)
Section 19A approved medicine
Fluorouracil Injection, USP 5g/100mL (50mg/mL) vial (Xiromed, USA)
Section 19A approval holder
ORSPEC Pharma Pty Ltd ABN 15 634 980 417
Phone
02 4339 4239
Approved until
Status
Current
Medicines in short supply/unavailable
FLUOROURACIL ACCORD fluorouracil 5000 mg/100 mL injection vial - ARTG 285802
FLUOROURACIL EBEWE fluorouracil 5000 mg/100 mL solution for injection vial - ARTG 166741
FLUOROURACIL EBEWE fluorouracil 5000 mg/100 mL solution for injection vial - ARTG 166741
Indication(s)
Fluorouracil is indicated alone or in combination for the palliative treatment of malignant tumours, particularly of the breast, colon or rectum; and in the treatment of gastric and pancreatic carcinomas. Fluorouracil should only be used when other proven measures have failed or are considered impractical.
Images