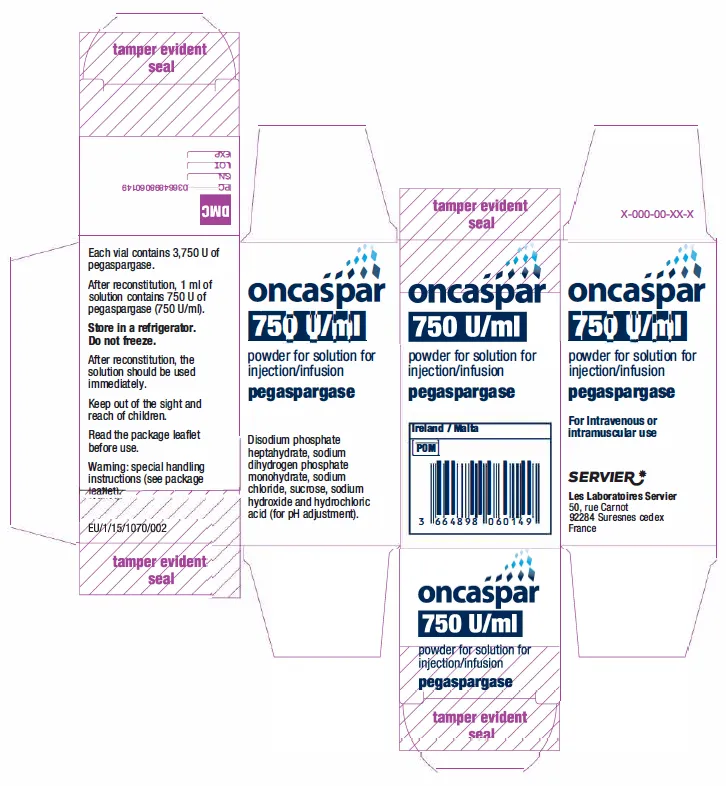
(Approval lapsed) ONCASPAR pegaspargase 3750 U/5mL (750 U/mL) powder for solution for injection/infusion (Ireland)
Section 19A approved medicine
(Approval lapsed) ONCASPAR pegaspargase 3750 U/5mL (750 U/mL) powder for solution for injection/infusion (Ireland)
Section 19A approval holder
Servier Laboratories (Aust.) Pty. Ltd ABN 54 004 838 500
Phone
1800 153 590
Approved until
Status
Expired
Medicines in short supply/unavailable
ONCASPAR pegaspargase 3750 units/5mL powder for solution injection/infusion vial - ARTG 303807
Indication(s)
ONCASPAR is indicated as a component of antineoplastic combination therapy in patients with Acute Lymphoblastic Leukaemia (ALL)
Images