Medical practices using the Medical Director software can download and install templates to their software to create Adverse Drug Reaction (ADR) reports.
Completed reports can be emailed to us at ADR.Reports@tga.gov.au, faxed to 02 6232 8392 or posted to us at: TGA, PO Box 100, Woden ACT 2606, Australia.
Before you start
This user guide assumes a working knowledge of Medical Director. For installation purposes, you will need to be connected to the Internet in order to download the template from our website.
Download the ADR Reporting template
The ADR Reporting template can be accessed at Medical Director: Adverse Drug Reaction Report template (rtf,542kb).
- Download the MD ADR Reporting Form.rtf from the TGA website and SAVE to the desktop.
Install the template in Medical Director
- Open Medical Director.
- Open the Letter Writer (F8).
- Select File; New (figure 1), then select 'Blank Template' (figure 2).
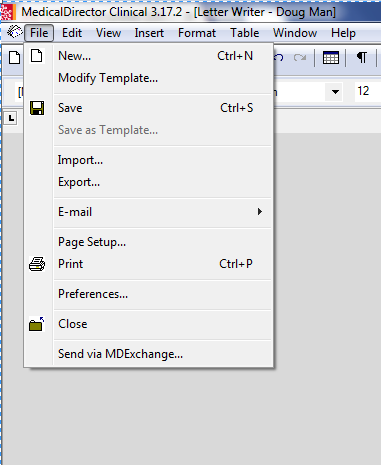
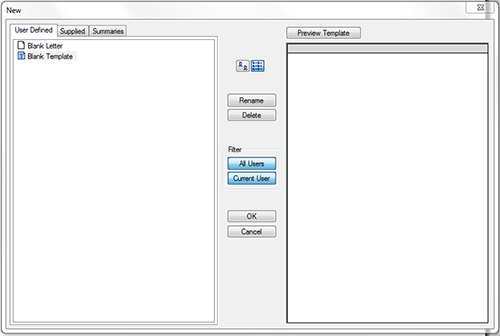
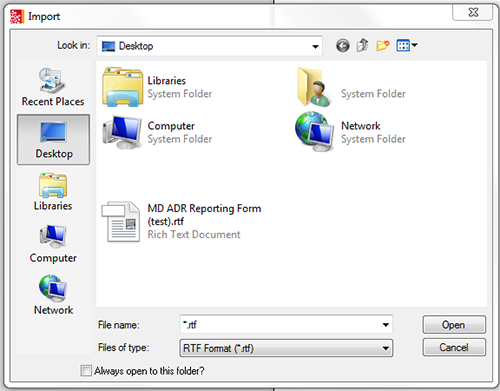
- Select 'File'; 'Import'; Select 'MD ADR Reporting Form.rtf' from desktop as shown in figure 3.
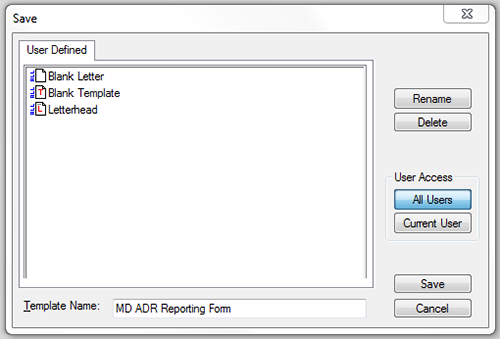
- Select File; 'Save as template…' and name the template 'MD ADR Reporting Form' and select 'Save' as shown in figure 4.
The template is now ready to use.
Use the template
A user guide is available for using the ADR Reporting template in Medical Director on our website.
| Version | Description of change | Author | Effective date |
|---|---|---|---|
| V1.0 | Original publication | Technical and Safety Improvement | October 2018 |
Print version
Product types

