Bulk packs supplied to consumers without child-resistant packaging
The TGA has become aware that some bottles of paracetamol intended only for pharmacist use may have been supplied directly to consumers. As these packs were not intended for direct supply, they do not have child-resistant packaging.
The affected product is APOHEALTH Paracetamol Pain Relief paracetamol 500 mg film-coated tablets (AUST R 230262) sold in bottles. The main label of the product includes the statement ‘For dispensing only. Not for patient supply’.
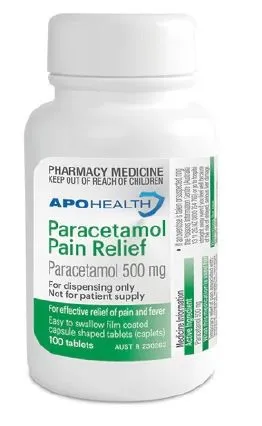
We are aware that this product may have been sold to some consumers either through physical pharmacies or online stores.
Check your bottle and label
Consumers who may have bought the product should check if they have it and, if so, ensure it is kept out of reach of children. There is a potential risk for an accidental overdose should a child be able to access the medicine in the original container.
If you have concerns, you should return the product to the place of purchase for advice.
A separate product, APOHEALTH Paracetamol Pain Relief paracetamol 500 mg film coated tablets in blister packaging (AUST R 230261), is not affected and is intended to be supplied directly to consumers.
Consumers should generally keep all medicines out of the reach of children.
If you suspect an overdose or unintended consumption of a medicine by a child, you should contact the Poisons Information Centre on 131 126 immediately.

