Consumers and health professionals should be aware that examples of counterfeit Roche labelled products have been stopped at the Australian border. These injectable products are labelled as Roche branded Laroscorbine Platinum containing Vitamin C, collagen and polynucleotides and may pose a serious risk to your health and should not be used.
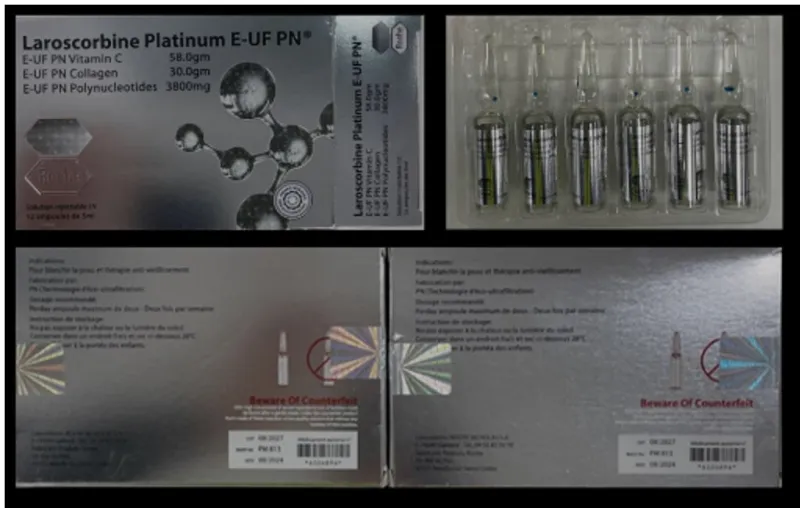
While these products appear genuine, Roche has confirmed they no longer manufacture Laroscorbine, and this, along with any product identified as Roche branded Laroscorbine is not genuine.
Consumers are advised that these counterfeit ‘Roche – Laroscorbine Platinum’ branded products have not been assessed by the TGA for quality, safety or efficacy as required under Australian legislation for therapeutic goods.
Consumers should be warned that manufacturers of counterfeit goods are producing products that, to the untrained eye, may appear legitimate, highlighting the need to purchase your medicines from legitimate sources using the lawful supply chain.
Only the approved supplier (sponsor), as entered on the Australia Register of Therapeutic Goods (ARTG), or their agent, can lawfully import cosmetic injectables into Australia for commercial supply. Supply includes sale of, administration to, or application in, the treatment of a person.
We advise consumers to exercise extreme caution when purchasing medicines from unknown overseas websites.
Products purchased over the internet:
- may be fake
- may contain incorrect or undisclosed and potentially harmful ingredients
- may not meet the same standards of quality, safety and efficacy as those approved by the TGA for supply in Australia.
For your safety, always buy medicines from reputable sources and consult your healthcare provider or local registered pharmacy if you have any concerns.
Counterfeit products cannot be imported under the Personal Importation Scheme. Knowingly importing, supplying and/or giving away counterfeit therapeutic goods is illegal and poses a significant public health and safety risk.
Information for consumers
- Stop using counterfeit ‘Roche - Laroscorbine Platinum’ and take any remaining product to your local pharmacy for safe disposal.
- If you have any concerns arising from your use of this product, consult your health care practitioner.
- If you suspect you have had a side effect (also known as an adverse event) to this or a similar medicine, report it to the TGA.
- If possible, keep the medicine as we may request it for testing.
- If you are considering purchasing medicines from overseas, watch this short video on the risks associated with buying medicines and medical devices online.
Action we are taking
We will notify ABF to seize and destroy any of these products intercepted at the border.
Report counterfeit medicines and medical devices
If you are worried about counterfeit medicines or medical devices, and want to report an issue, you can report the matter to the TGA.
| Phone: | 1800 020 653 |
| Online: | Report a problem or side effect |
| Email: | info@tga.gov.au |

