If you have the following devices you may be affected.
- Heartstart HS1
- Heartstart Onsite
- Heartstart Home
Philips Electronics Australia Ltd, in consultation with the Therapeutic Goods Administration, is undertaking a Product Defection Correction to alert users to a possible problem with the AED SMART Pads used with these defibrillators.
What is the problem?
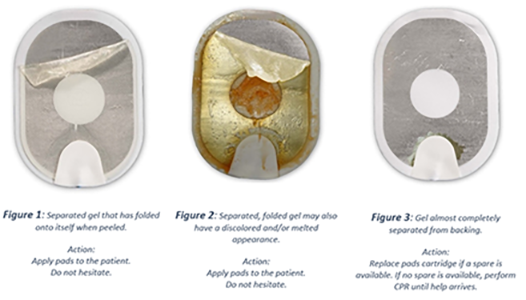
The skin contact gel may separate from the device's defibrillation pads which can lead to less effective or ineffective delivery of therapy.
What should you do?
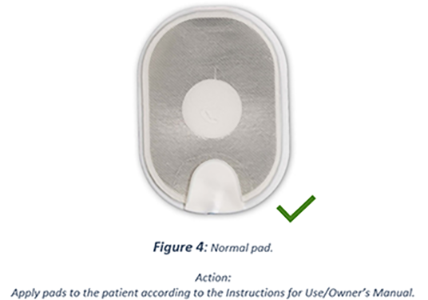
If any gel is still present on the pad after the backing is peeled off, use as normal. Sometimes the gel is discoloured but this doesn't affect it, use as normal.
If no gel is present, use a replacement cartridge if you have one or continue CPR until help arrives.
What is Philips doing?
Philips has advised its customers to:
- Store a spare SMART Pads cartridge with your HS1/OnSite/Home AED.
- Never tamper with the foil seal to examine the pad's gel prior to use. Pads will quickly dry out if the foil seal is broken.
- Continue using the device as per instructions.
- No matter the state of the pads, follow the voice prompts - AED devices talk users through the necessary actions.
- During use, ensure most of the pad surface is covered with gel and apply the pads to the patient.
- If gel separates during pad preparation, ensure it covers as much of the pad as possible and does not fold onto itself, as this would reduce the available surface area for therapy delivery.
- If gel has almost completely separated from the pad, replace with spares if available and continue.
- If spare cartridges are unavailable, provide CPR until emergency medical services personnel arrive.
This problem has been seen in pads from multiple manufacturing lots so design changes are being developed.
Philips will provide redesigned replacement SMART Pads to all end users when these are available.
Philips has received 115 complaints since 2010 (of which 84 complaints were received in 2021).
What are AED SMART Pads used for?
SMART Pads are used exclusively in HS1 Home and OnSite defibrillator models M5066A and M5068A. These are automated external defibrillators (AEDs) used in cases of life-threatening irregular heartbeat (also known as cardiac arrhythmia or cardiac dysrhythmia) that leads to cardiac arrest. Defibrillators work by applying electricity to stop the arrhythmia, allowing the heart to re-establish an effective rhythm.
If you have any questions or concerns about this issue, contact Philips Service Delivery team on 1800 251 400.
Reporting problems
Consumers and health professionals are encouraged to report problems with medical devices. Your report will contribute to the TGA's monitoring of these products. For more information see the TGA Incident Reporting and Investigation Scheme (IRIS).
The TGA cannot give advice about an individual's medical condition. You are strongly encouraged to talk with a health professional if you are concerned about a possible adverse event associated with a medical device.

