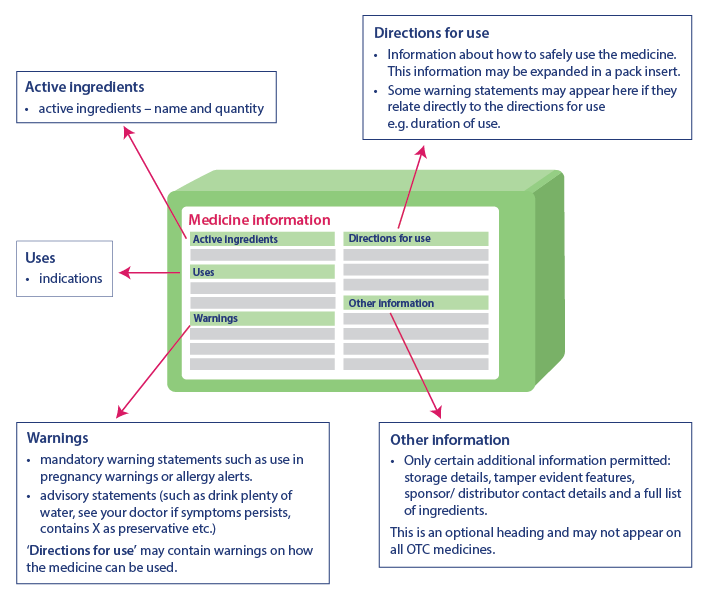
Labelling changes: information for health professionals
Clearer medicine labels
Published
Related content
-
Before you take the red pill or the blue pill, check the medicine label for nuts
What does Neo from the Matrix need to know before choosing a pill to take? -
Changes to ingredient names: End of transition period - are you ready?
The transition period for updating medicine ingredient names ends on 30 April 2020. -
Guidance changes are coming soon
Some changes are rolling out soon to improve your experience using guidance on our website. We’re aiming to make our content clearer, more accessible and easier to navigate.