We are implementing new rules that require medical devices to be identified using a Unique Device Identifier (UDI).
The UDI is a globally unique identifier that is shown on device labels, the device’s packaging and, sometimes, directly marked on the device itself.
These identifiers will be stored in the Australian UDI Database, which you can access for free. The AusUDID will contain the device identifier, as well as other useful information about the device such as:
- Brand name
- Manufacturer information
- Clinical information.
You can use the AusUDID to look up devices you use or are getting implanted.
The AusUDID does not store patient information.
UDI can strengthen patient safety by:
- providing consumers with better information on medical devices and their characteristics
- helping identify medical device problems for specific devices sooner
- faster and more effective recalls
- offering a way for consumers to record in their own records (MyHealth record), information about the device.
UDI overview
UDIs are like barcodes for products. It combines numbers, symbols and letters that identify the product. The UDI will be unique to every model of device. It will be issued by a TGA recognised Issuing Agency to ensure that it is globally consistent and unique for each type of medical device.
This includes medical devices manufactured overseas and supplied in Australia.
Devices with a UDI
Most medical devices and in vitro diagnostic (IVD) devices supplied in Australia will have a UDI. We are introducing these rules in a phased approach, meaning some devices will have UDIs before others.
High risk, implanted, and diagnostic test medical devices will need to comply with the UDI regulations first, followed by other risk classes.
Low risk medical devices will not need to comply with the UDI regulations. They will continue to be identified by existing methods.
Medical devices with higher risk classifications need to comply with the UDI regulations:
Types of devices | Device classification and risk | UDI required |
|---|---|---|
| Class I (low risk) | No |
| Class Im (low-medium risk) | No |
| Class Is (low-medium risk) | Yes |
| Class IIa (low-medium risk) | Yes |
| Class IIb (medium-high risk) | Yes |
| Class III (high risk) | Yes |
In vitro diagnostic (IVD) devices with the following classifications need to comply with the UDI regulations:
Types of devices | Device classification and risk | UDI required |
|---|---|---|
| Class 1 (no public health risk or low personal risk) | Selected devices |
| Class 2 (low public health risk or moderate personal risk) | Yes |
| Class 3 (moderate public health risk or high personal risk) | Yes |
| Class 4 (high public health risk) | Yes |
More information including detailed definitions of in vitro diagnostic devices is available at Overview of medical devices and IVD regulation.
UDI labels
UDI labels will look similar to existing labels. However, they contain specific information and follow special rules to ensure that the device can be tracked and traced.
An example of a UDI label incorporated into existing labelling.

A UDI will be applied to the device labelling or packaging in a format that allows it to be read by humans and machines.
There are no restrictions on the form for the machine-readable portion of a UDI so you might see a UDI in the various forms such as a barcode or data matrix.
Australian UDI Database (AusUDID)
The AusUDID stores data about medical devices supplied in Australia, including the UDI, brand name, size, clinical characteristics and any related Patient Information Leaflets (PILs).
The AusUDID allows you to search, view, and download information at no cost.
AusUDID does not store patient information.
Accessing AusUDID
To find out more about AusUDID, see Australian UDI Database.
UDI for adverse events and recalls
When they are available, UDIs will be included in official reporting for medical devices, for example in:
- market actions such as recalls
- reporting of adverse events.
To find information about Australian market actions see Market actions.
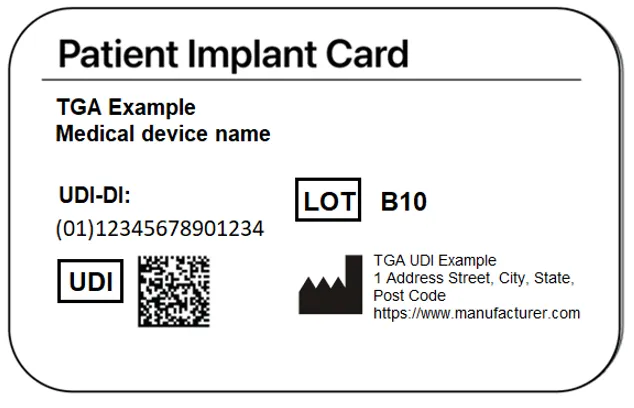
UDI for Patient Implant Cards (PICs)
When you have a medical device implanted in a hospital or health care setting in Australia, you should be provided with a Patient Implant Card (PIC). The PIC contains important information about your medical implant including the UDI.
Using the UDI on your PIC, you can search the AusUDID to find more details about the device.
Once the UDI is included on a Patient Implant Card, it will be on the card in both a machine readable and human readable format, as in the image below.
Contact us
Email: UDI@health.gov.au.

