Note
To help healthcare organisations adopt UDI into their processes, see:
UDI for Australian healthcare: Executive summary
Overview of Unique Device Identification for Australian Healthcare
UDI in healthcare
The Australian Unique Device Identification (UDI) system is a globally harmonised system that can support improved tracking and tracing of medical devices including within the Australian healthcare system.
The Unique Device Identifier (UDI) is intended to be the key identifier used in administrative and clinical transactions.
Examples include:
- in discharge summaries
- patients’ records
- registries
- clinical notes and records
- purchase orders
- reimbursement or claims documentation
- invoices
- inventory maintenance/management.
Benefits of adopting the UDI can contribute to a more reliable and efficient system of tracking and tracing medical device issues, as well as ensuring correct products are used.
A UDI can also be stored within electronic health records and will be required on Patient Implant Cards (PICs), adverse event reporting, device incident reporting and when undertaking market actions.
If implemented across the Australian healthcare system, UDI will offer benefits for patients, healthcare facilities, research facilities and the supply chain, such as:
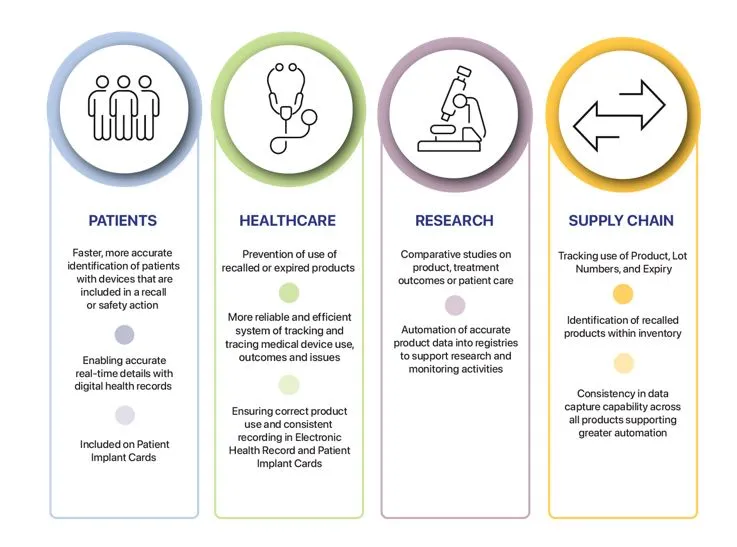
Unique Device Identifier overview
A UDI is a combination of numbers, symbols and letters used to identify the model of device.
A UDI will be present on the device label or the device itself, as well as all applicable levels of device packaging. It will not replace any existing labelling.
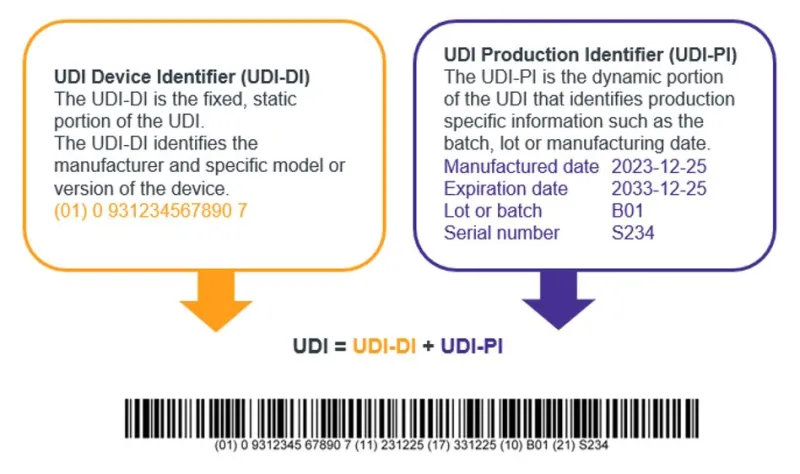
A UDI is made up of 2 parts:
- UDI Device Identifier (UDI-DI)
- UDI Production Identifier (UDI-PI).
The UDI-DI part of the UDI is used to identify the model of device. It is also the ‘access’ key to information stored in the Australian UDI Database (AusUDID).
The UDI-PI part of the UDI is used to identify production information of the device. It may include information such as the batch number, lot number or expiry dates.
The UDI-PI is not included in the AusUDID.
The full UDI is applied to a device or the device’s packaging in 2 forms:
- Human readable
- Machine readable.
This is known as the UDI Carrier.
You may see different kinds of UDI Carrier, such as:
- Linear barcodes
- Data matrix barcodes.
Purpose of UDI
The UDI system is a key pillar for the post-market surveillance and monitoring of medical devices.
The establishment of the UDI system is one of the medical device reforms as outlined in An Action Plan for Medical Devices.
The main purpose of the UDI system is to improve patient safety.
When adopted in the supply chain, clinical and other health systems, UDI can enable easier and faster identification of medical devices, supporting:
- removal of those medical devices from storage and distribution to prevent further use
- faster identification of medical devices implanted into patients in the event of an adverse event, or market action.
It also allows patients, consumers, and health professionals to access product information in the AusUDID about the medical devices that they use.
The AusUDID provides an easy to access and consistent location for up-to-date product information.
UDI could improve medical device performance assessment by regulatory bodies, clinical quality registries and medical device manufacturers through accurate product identification that better supports comparative studies.
UDI implementation in hospitals and hospital software products
We have published an implementation document to support healthcare facilities to better understand UDI and to assist their consideration of adopting the UDI system.
See the Overview of Unique Device Identification for Australian Healthcare.
Implementation or adoption of UDI in hospitals and healthcare organisations, or the software products used in a healthcare organisation is a consideration and decision by those facilities.
We are communicating progress to the Australian Commission on Safety and Quality in Health Care, the public and private hospital sectors, procurement teams and software vendors.
Devices required to meet UDI requirements
Medical devices and in vitro diagnostic (IVD) devices supplied in Australia require a UDI, unless otherwise exempt from these requirements.
Compliance with UDI requirements is being phased in according to the risk classification of the device.
UDI requirements apply to medical devices and IVD devices with the following classifications:
Medical device classes
- Class III
- Class IIb
- Class IIa
- Class Is – supplied sterile
IVD classes
- Class 4
- Class 3
- Class 2
- Class 1 typically categorised as:
- Instrument/analyser (GMDN Collective Term 943)
- Software (GMDN Collective Term 944)
Some medical devices already have a UDI on the device, the labelling or packaging due to UDI being implemented globally.
We accept UDI compliant labels from the United States (US) and the European Union (EU).
Understanding UDI Carriers
UDI Carriers may come in different forms, as some kinds may be more appropriate for specific settings.
The below image is an example of a UDI Carrier in the form of a barcode.
We will publish further resources on understanding UDI Carriers.
UDI reporting by healthcare providers
If a UDI is available, it must be included on Patient Implant Cards (PICs) and notifications to us including adverse events, incident reports and market actions.
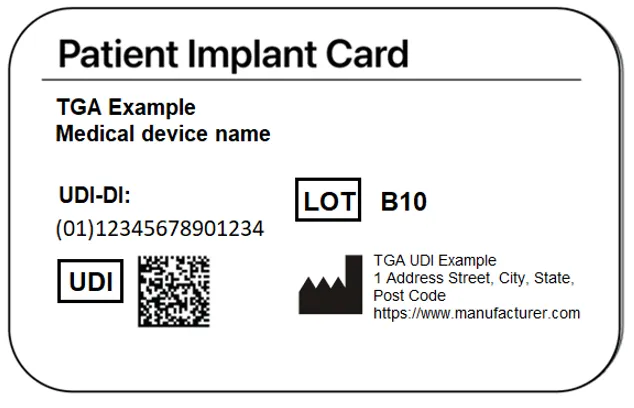
UDIs on Patient Implant Cards (PICs)
Patient Implant Cards (PICs) are provided to patients who receive a medical device implantation.
The UDI must be recorded on the PIC for all implantable devices, with the full UDI (UDI-DI and UDI-PI) in machine-readable form, and UDI-DI in human readable form.
PICs currently require the following information to be supplied:
- name of the device
- model of the device
- batch code, lot number or serial number of the device
- manufacturer’s name, address, and website.
The UDI-PI will either being shown as a single field or split into the above data elements.
An example of a PIC with a UDI is shown below.
UDIs will not be required on Instructions for Use documentation, although some manufacturers may choose to do so.
For more information on PICs visit Patient Implant Cards and information leaflets.
Australian UDI Database (AusUDID)
You can access the AusUDID here: TGA AusUDID.
We have established the AusUDID as a repository of UDI-DIs and related information for devices supplied in Australia.
The data in the AusUDID links to relevant inclusions in the Australian Register of Therapeutic Goods (ARTG).
You can search, view and download UDI records using criteria such as the UDI-DI, brand name, manufacturer name, sponsor name or a GMDN code.
The AusUDID allows you to access Patient Information Leaflets for a specific device if the sponsor or manufacturer has attached this information for their device.
For more information on using the AusUDID, see Australian UDI Database.
Contact us
Email UDI@health.gov.au
Page history
- Added links to:
- Overview of UDI for Australian healthcare
- TGA AusUDID
- Updated the image of a Patient Implant Card example
- Added links to:
- Overview of UDI for Australian healthcare
- TGA AusUDID
- Updated the image of a Patient Implant Card example

