A Medical Device Production Systems (MDPS) is an end-to-end system to produce medical devices.
It consists of all components needed to make medical devices, including the raw materials, design and manufacturing equipment, and the instructions for operating the system.
The medical devices produced by a system also form part of the MDPS.
For a system to be an MDPS, it needs to be used by a health professional, or a suitably qualified person within a healthcare facility.
Key features of a Medical Device Production System
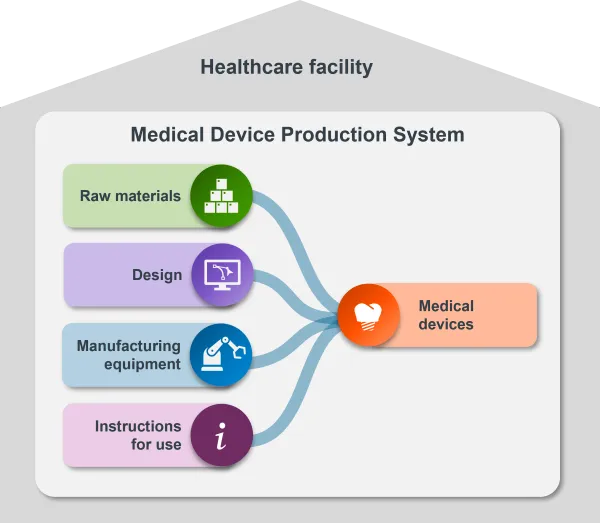
This infographic illustrates the key features of a Medical Devices Production System.
An MDPS is an end-to-end system to produce medical devices.
It consists of all components needed to make medical devices, including the raw materials, design and manufacturing equipment, and the instructions for use.
The medical devices produced with it also form part of the MDPS.
For a system to be an MDPS, it needs to be used by a health professional, or a suitably qualified person within a healthcare facility.
Considerations for MDPS manufacturers and users
The MDPS concept is intended to offer a new regulatory path for point-of-care manufacturing of medical devices.
An MDPS user is not considered the manufacturer of the medical devices it produces, if:
- The MDPS is included in the Australian Register of Therapeutic Goods (ARTG); and
- The user follows the instructions of the MDPS manufacturer.
MDPS manufacturers:
- Specify the intended use, raw materials, components and instructions for the MDPS.
- Specify the intended use of the medical devices produced with the MDPS.
- Comply with Essential Principles and apply conformity assessment procedures.
- Obtain a single ARTG entry. This will cover all MDPS components and the devices it is intended to produce.
- Supply their MDPS to healthcare facilities.
If the MDPS is not used according to the manufacturer’s intended purpose or instructions, the user becomes responsible for the devices produced. For example, if the user:
- sources a raw material from a supplier not recommended by the MDPS manufacturer;
- does not follow calibration or servicing instructions; or
- is not trained in the way specified by the MDPS manufacturer;
then the MDPS manufacturer is not responsible for the devices produced. Instead, the user is the legal manufacturer of those devices.
MDPS users in healthcare facilities:
- Do not need to include the MDPS-produced medical devices in the ARTG.
- Can be assured of the quality and performance of devices produced by the ARTG-included MDPS.
- Make devices at the point-of-care, thereby providing devices faster to their patients.
- Improve inventory control.
MDPS examples
Example 1: Ceramic milling system to produce patient-matched dental crowns
A manufacturer develops a system to be sold to dental clinics. It consists of:
- an intraoral scanner;
- CAD/CAM software that:
- reads files generated from intraoral scans;
- designs a dental crown for a particular patient according to the scans; and
- generates files to control the milling machine.
- ceramic blocks;
- a ceramic milling machine;
- a furnace for firing; and
- post-machining finishing equipment.
Example 2: 3D-printing system to produce patient-matched orthopaedic splints
The company Splint Solutions makes patient-matched splints in a lab. Splint Solutions wants to move some manufacture to healthcare facilities.
Splint Solutions develops a system to sell to orthopaedic departments.
This system produces patient-matched splints at the point-of-care and consists of:
- CAD/CAM software;
- a hand-held colour 3D scanner;
- thermoplastic polyurethane; and
- a 3D printer.
Counter-example: Not an MDPS
The company BoneHeal develops a system to 3D-print titanium implants used in bone reconstruction.
This system is intended to be sold to hospitals and consists of:
- CT image-processing software;
- CAD/CAM software;
- finite element analysis software;
- titanium powder; and
- a metal printer.
BoneHeal trains hospital staff to use the system to design and print patient-matched implants.
The implants are then sent back to BoneHeal for post processing.
BoneHeal sends the processed implants back to the hospital to be used.
This system does not meet the definition of an MDPS because the post-processing step takes place outside the hospital.
It is therefore not a complete end-to-end system to produce medical devices.
How to supply an MDPS
The MDPS is a new regulatory concept. In the future, manufacturers and/or sponsors may be able to include an MDPS in the ARTG.
For this to happen, a legislative instrument will need to be established.
If you are considering manufacturing an MDPS, request a pre-submission meeting to discuss your specific circumstances.
Related links
Page history
Original publication
Original publication

