Some medicines may contain substances that might cause an allergic reaction in some people. Unlike foods, most medicines are not required to list all their ingredients on the label. The active ingredient will always be on the medicine label, but only some inactive ingredients, also called excipients, must be on the label.
In the first instance, you should talk to your doctor, pharmacist or health professional if you have questions or concerns about allergies or the contents of your medicine.
Medicine labels are also being improved to make it easier for you to find important information. These changes will mean that over time more medicines will be labelled with information on more allergens. You can search the list below for potential allergens that must be declared.
There are a number of other ways to find information on ingredients in a medicine, including contacting the medicine supplier - their details must be provided on the label, reviewing the Consumer Medicine Information or in the public medicine summary.
Note that some potential allergens, such as impurities from manufacturing, may not be included in this information or declared.
For more information see the 'I can't find what I'm looking for!' tab and 'What ingredients are in my medicine?'
Better Medicine Labels
Medicine companies were given time to redesign their labels to meet new rules . While labels are changing, it is important to ask your doctor, pharmacist or other health professional about food allergen content in medications.
Most medicines should be meeting the new label rules, but there may be old labels on some existing stock. Also, some medicine companies were not able to meet the new rules in time due to the impacts of COVID-19 and asked for consent to supply medicines with old labels for a short period.
What is new on medicine labels?
Under the new rules, there will be a longer list of substances that must be declared on a label if they are present in the medicine. The additional substances include crustacea, fish, eggs, soya, milk and tree nuts. Some ingredients that may be allergens, such as peanuts and gluten, must already be declared on medicine labels.
However, you may have allergies to substances which do not have to be on medicines labels under Australian law. If you are allergic to a substance and want to know if it must be declared on a medicine label, you can search the list below for potential allergens that must be declared.
Under the new rules, prescription medicine labels will have either a declaration about the allergen or a statement directing you to the Consumer Medicine Information leaflet. Before these rules, prescription medicines did not need to declare this allergen information on the label.
Where can I find allergen information on the label
It can sometimes be difficult to tell whether a medicine label is following the old rules or the new rules. Therefore, it is important to keep asking your doctor, pharmacist or other health professional about food allergen content in medications.
You will usually see allergens that must be declared on the label as "contains (substance name)".

Prescription medicines
Under the new rules for prescription medicines, allergen information must appear on either the label on the pack or in the Consumer Medicine Information leaflet. If it is in the leaflet, there will be a prompt on the pack telling you this. There is no specific place on the medicine pack that this allergen information or statement must appear.

Over-the-counter medicines
For most over-the-counter medicines, the new rules mean there will be a table of critical health information on the back or side of the medicine pack. Information about potential allergens will be listed under the "Warnings" heading. This will always appear after information about the uses for your medicine. There may also be an "allergy" subheading under "Warnings".
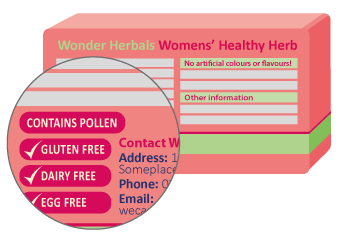
Listed medicines (includes most complementary medicines)
There is no specific place on listed medicine packs where allergen information must appear, however it is usually on the back or side label.
I can't find what I'm looking for!
In the first instance, you should talk to your doctor, pharmacist or health practitioner if you have any concerns about the contents of your medicine.
There are also other ways to find information about the contents of a medicine:
- Many medicines have a Consumer Medicine Information leaflet (CMI). Search 'CMI' on the TGA website.
- If your medicine has been prescribed to you, your doctor or pharmacist might also look in the Product Information (PI) which can be found on the TGA website for prescription medicines.
- Check the medicine's Australian Register of Therapeutic Goods (ARTG) public summary on the TGA website. You can find your medicine's ARTG summary by searching using the name of the medicine or its AUST R or AUST L number. The summary will contain information about the ingredients in the medicine formulation, but not:
- manufacturing impurities or contaminants
- ingredients within flavour, fragrance or colour mixtures
- components of an ingredient (such as caffeine in a herbal extract)
the source of an ingredient (such as whether an ingredient is of animal origin, or whether it is natural or synthetic).
See also 'How to find ingredient information' on the 'What ingredients are in my medicine' page.
- You can contact the medicine sponsor or distributor. Their contact details must always be on the medicine label.
- Contact the TGA to ask if a particular substance is an ingredient in a medicine. You will need to tell us the name of the substance (or substances) you want to know about, the name of the medicine and the AUST L or AUST R number.
- We hold information about medicine ingredients, but not manufacturing impurities or contaminants.
Search the list of allergens that will be on medicine labels
The following is a list of substances that must be declared on labels and includes potential allergens. Most medicines should be meeting these new labelling rules, but there may be old labels on some existing stock that do not declare the new substances.
Rules about how and what substances are declared
This is a list of all the substances that are required under new labelling rules to be declared on medicine labels. Many of these substances are included because they are allergens.
There are rules about when substances need to be declared on medicine labels. For example:
- some substances don’t have to be declared if the medicine is only for topical use
- some substances are only declared if there is a certain amount in the medicine
Substances that were not previously required to be declared on medicine labels are shown in the table below as a "new substance".
To search, start typing what you are looking for into the search field below. To reset the search, simply delete your search terms from the field. You can also sort on any of the columns.
| Ingredient* | New substance | Route of administration | Label statement | Other information |
|---|---|---|---|---|
| antibiotics | Yes | All | "contains residual 'antibiotic name'" | This declaration is only required when the antibiotic is not an active ingredient and is only a residue. |
| aspartame | No | Oral only | "contains aspartame" | |
| benzoates | No | All | "contains benzoates" | Examples include benzoic acid and sodium benzoate. |
| crustacea and crustacean products | Yes | All | "contains crustacea" or "contains crustacean products" | Examples include crab, crayfish, lobster, prawn and shrimp. |
| egg and egg products | Yes | All | "contains egg", or "contains egg products", or "manufactured in eggs" | Examples include dried egg yolk, egg lecithin and the influenza vaccine. |
| ethanol | No | All | "contains alcohol" | If the concentration is 3% v/v or more, the quantity of ethanol as %v/v will be declared on the label. |
| fish and fish products | Yes | All | "contains fish", or "contains fish products" | Examples include freshwater fish, diadromous fish and marine fish, including cod, cod – liver oil, halibut, shark and tuna. |
| galactose | No | Oral only | "contains galactose" | |
| gluten | No | All, except for skin and mucous membrane applications. | "contains gluten" | Includes gluten that is present naturally as a part of an ingredient such as wheat starch. Gluten is only required to be declared on the label where the concentration in the medicine is 20 parts per million or more. |
| hydroxybenzoic acid esters | No | All | "contains hydroxybenzoates" | Examples include ethyl hydroxybenzoate, methyl hydroxybenzoate, propyl hydroxybenzoate, sodium ethyl hydroxybenzoate, sodium methyl hydroxybenzoate and sodium propyl hydroxybenzoate . |
| lactose | No | Oral only | "contains lactose" | If the lactose is derived from milk, the label will not need to have the "contains milk products" declaration. |
| milk and milk products | Yes | All | "contains milk", or "contains milk products" | Examples include casein, hydrolysed milk protein, non-fat dry milk, whey powder and whole dry milk. If lactose is the only milk product that the medicine contains, the label will only need to have the "contains lactose" declaration. |
| peanut and peanut products | No | All | "contains peanuts", or "contains peanut products" | Examples include Arachis hypogea and arachis (peanut) oil. |
| phenylalanine | No | All, except for skin and mucous membrane applications. | "contains phenylalanine" | |
| pollen | No | Oral only | "contains pollen" | |
| potassium salts | Yes | Oral only | If the total maximum daily dose of the medicine contains more than 39mg, the label must declare the amount of potassium in each tablet, capsule or in a stated amount of a solid or liquid. "contains XX mg of potassium per dose"* | Examples include potassium bicarbonate and potassium chloride. |
| propolis | No | Oral only | "contains propolis" | |
| royal jelly | No | Oral only | "contains royal jelly" | |
| saccharin | No | Oral only | "contains saccharin" | Examples include saccharin calcium and saccharin sodium. |
| sesame seeds and sesame seed products | Yes | All | "contains sesame seed", or "contains sesame seed products" | Examples include Sesamum indicum, sesame seed and sesame oil. |
| sodium salts | No | Oral only | If the total maximum daily dose of the medicine contains more than 120mg, the label must declare the amount of sodium in each tablet, capsule or in a stated amount of a solid or liquid. "contains XX mg of sodium per dose"* | Examples include sodium bicarbonate and sodium chloride. |
| soya and soya bean products | Yes | All | "contains soya bean", or "contains soya bean products" | Examples include Glycine max, soya bean soya oil. Does not include fully refined soya oil, vitamin e (such as d-alpha tocopherol) from soya bean sources, phytosterols from soya bean sources and plant stanol esters from soya bean sources. |
| sorbates | No | All | "contains sorbates" | Examples include potassium sorbate and sorbic acid. |
| sucralose | Yes | Oral only | "contains sucralose" | |
| sugar alcohols | No | Oral only | "contains sugar alcohols" or "contains name of sugar alcohol" If the total maximum daily dose of the medicine contains more than 2g, the label must declare the amount of sugar alcohols present per recommended maximum daily dose; and a statement "Products containing (name of sugar alcohol) may have a laxative effect or cause diarrhoea" | Examples include erythritol, isomalt, lactitol, maltitol, mannitol, polydextrose, sorbitol and xylitol. |
| sugars - monosaccharides and disaccharides | No | Oral only | If the total maximum daily dose of the medicine contains more than 100mg of sugar, resulting in a significant glycaemic effect, the label must declare sugar as "contains sugars" | Examples include fructose, glucose, honey, invert sugar, lactose, maltose and sucrose. |
| sulfites | No | All | "contains sulfites" | Examples include potassium metabisulfite, sodium bisulfite, sodium metabisulfite, sodium sulphite and sulphur dioxide. If a medicine may contains sulfur dioxide as a residue, for example, gelatin, it must be identified on the label. |
| tartrazine | No | All | "contains tartrazine" | |
| tree nuts and tree nut products | Yes | All | "contains tree nuts", or "contains tree nut products" | Examples include: Juglans nigra, Macadamia ternifolia, Prunis dulcis, almond oil, macadamia nut oil almond, Brazil, cashew, chestnut and walnut. Tree nuts are the seeds of a variety of trees and shrubs which are characterised by a hard inedible shell enclosing an oily seed. Coconut is the fruit of the palm (Cocos nucifera) and is not considered to be a tree nut. |
* This list comes from Schedule 1 to Therapeutic Goods Order No. 91 - Standard for labels of prescription and related medicines and Therapeutic Goods Order No. 92 - Standard for labels of non-prescription medicines.
Related content
-
Inactive ingredients, the unsung players in medicines
BlogHow to find information about the inactive ingredients in your medicines. -
Before you take the red pill or the blue pill, check the medicine label for nuts
BlogWhat does Neo from the Matrix need to know before choosing a pill to take? -
Labelling changes: information for health professionals
News articlesMedicine labels started to change in 2020 to be more consistent, highlight key information and align with medicine labels used in other countries.



