How we assess the quality of vaccines
We ensure there is an independent quality assessment for every batch of vaccine supplied in Australia. This page provides information about the batch release assessment process for vaccines supplied and available in Australia.
During the evaluation process for registration, we review data to confirm that the manufacturing process is well controlled. This sets up how the quality of the vaccine will be maintained in future batches. Further information on the pre-registration process is available at Vaccines Overview.
Batch release assessment
Our batch release program ensures there is an independent quality assessment of every batch of vaccine supplied in Australia. This is to assure that vaccine quality is maintained following registration.
That means every vaccine batch must pass assessment before it is released for use. This can happen based on overseas certification (Pathway 1) or TGA laboratory assessment (Pathway 2). We may test vaccines released under either pathway.
Pathway 1 - Release based on overseas certification
This pathway relies on overseas certification to show the batch has been independently tested by an accredited National Control Laboratory, such as the Official Control Authority Batch Release (OCABR) process in Europe.
The OCABR process involves laboratory testing and assesses the manufacturing protocol documents. Laboratory testing may cover potency or content, identity, integrity, appearance, and purity.
When the Sponsor of the vaccine provides evidence that the batch to be supplied in Australia has passed OCABR assessment and testing, we can release that batch of vaccine relying on the OCABR assessment and testing. Although released on OCABR certification, our laboratories may still test these batches as part of our batch release program. The sponsor must still supply samples, batch details and evidence maintaining acceptable shipping conditions for the batch under this pathway.
The availability of an OCABR certificate for vaccine batches entering Australia depends on various factors, including the intended destination of the batch. If an OCABR certificate is not available, Pathway 2 is used.
Pathway 2 - Release based on TGA assessment.
This pathway is used when no OCABR certificate is available.
Vaccine assessment using Pathway 2 is based on our own assessment of the manufacturing documentation (including testing results) and shipping information. Samples from every batch of vaccine supplied must be submitted to us. Testing may be carried out by the TGA Laboratories in line with our risk-based approach.
Pathway 1 Overseas certification and Pathway 2 TGA assessment
Flowchart of pathway 1. Overseas certification and pathway 2. TGA assessment
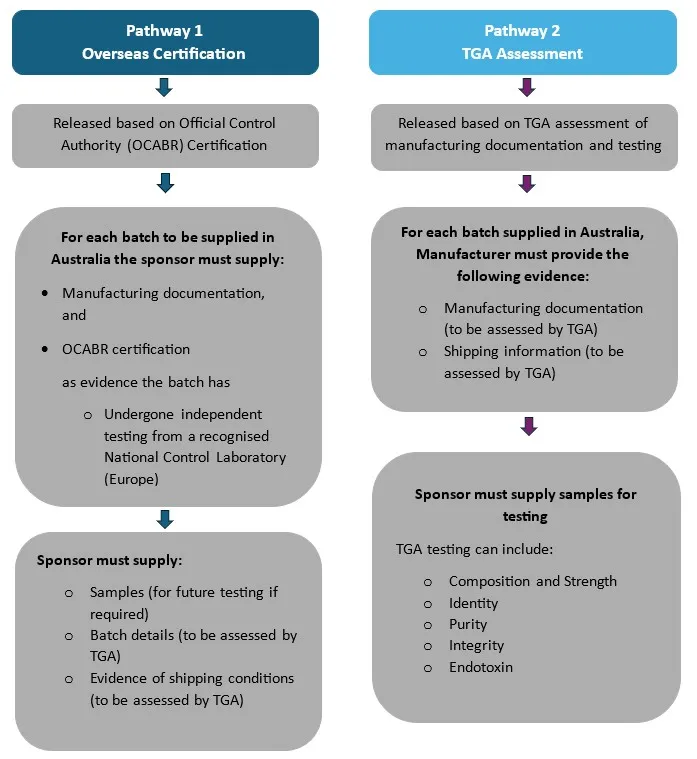
This flowchart illustrates two pathways for batch release of products supplied in Australia:
Pathway 1: Overseas Certification
- Basis for Release: Official Control Authority Batch Release (OCABR) Certification.
- Requirements for Each Batch:
- Sponsor must provide:
- Manufacturing documentation.
- OCABR certification as evidence that the batch has:
- Undergone independent testing by a recognized National Control Laboratory (Europe).
- Sponsor must provide:
- Additional Sponsor Obligations:
- Supply samples (for future testing if required).
- Provide batch details (to be assessed by TGA).
- Provide evidence of shipping conditions (to be assessed by TGA).
Pathway 2: TGA Assessment
- Basis for Release: TGA assessment of manufacturing documentation and testing.
- Requirements for Each Batch:
- Manufacturer must provide:
- Manufacturing documentation (to be assessed by TGA).
- Shipping information (to be assessed by TGA).
- Manufacturer must provide:
- Additional Sponsor Obligations:
- Supply samples for TGA testing.
- TGA testing may include:
- Composition and strength.
- Identity.
- Purity.
- Integrity.
- Endotoxin.
Key Difference:
- Pathway 1 relies on overseas certification and independent testing in Europe.
- Pathway 2 involves direct TGA assessment and testing within Australia.
Testing the vaccines
We undertake a risk-based assessment to determine if a vaccine is to be tested. Testing capabilities covering molecular, immunobiological, biochemical and in vitro testing methods have been developed to support quality assessment of vaccine covering:
Composition and strength
Potency
Identity
Purity and integrity
- Endotoxin
- Uniformity and size of vaccine components
COVID-19 vaccine batch release
In line with the batch release process established for all vaccines entering Australia, COVID-19 vaccines are assessed and released under either Pathway 1 or Pathway 2.
Information on TGA Laboratories testing results for COVID-19 vaccines
We have been performing batch release and testing on all COVID-19 vaccine batches that have been supplied in Australia. This process is a critical part of our regulatory oversight to ensure vaccine quality.
As Australia transitions out of the emergency phase of the pandemic, we will no longer publish batch testing results. See reporting adverse events if you have concerns over a batch you have received, or would like to report an adverse event following vaccination.
A Summary report for Batch release testing on mRNA vaccines is available. This report outlines batch release testing for mRNA vaccines supplied in Australia from February 2021 to July 2024.
A Summary report of residual DNA and endotoxin on CoVID-19 mRNA vaccines is also available. This report outlines the details of residual DNA and Endotoxin tests undertaken by the TGA Laboratories for mRNA vaccines.
Further, we are aware of misinformation in recent media and online reports that claim the COVID-19 mRNA vaccines are contaminated with excessive levels of DNA. Further information is available at Addressing misinformation about excessive DNA in the mRNA vaccines

